The Ministry of Health June 22 held a ceremony in Hanoi to receive the World Health Organization's formal certification of made-in-Vietnam vaccine.
Speaking at the ceremony, Deputy Prime Minister Vu Duc Dam said that WHO's formal certification of Vietnamese-made vaccine is a big event and a giant progress of the country's health sector in particular and the nation's science industry in general. This also manifests the Ministry of Health’s huge efforts and management agencies and scientists' hard work.
Mr. Dam continued that the Southeast Asian country’s regulatory system for vaccines officially certified by WHO as complying with international standards is a really good start of a long road of scientific research; accordingly, the health sector and scientists should continue having researches of vaccine to produce more high-quality vaccine to meet international standards in order to protect people’s health especially Vietnamese children.
He added the Ministry of Health should invest much on vaccine researches and promote commerce to develop and widen markets to sell made-in-Vietnam vaccine.
Dr. Shin Young-soo, Regional Director of WHO’s Western Pacific Region, said that Vietnam now has a fully-equipped national regulatory system that ensures the safety and effectiveness of vaccines they produce and use; accordingly, this is a great result for the regulators, but an even better result for the people of Vietnam, because it confirms that vaccines produced in the country are quality assured to international standards of production, safety, and effectiveness.
This accomplishment should serve as an inspiration to other countries in the Region and the world, said Dr. Shinh Young-soo.
According to WHO, Vietnam is facing a big challenge in developing vaccine industry sector, although if the country can produce vaccine for domestic market or for 1.7 million newborn babies every year under the National Expanded Immunization Program, made-in-Vietnam vaccine will have chance to export.
Moreover, with this achievement, nations in the world will pay more attention to manufacturing vaccine in the Southeast Asian nation where have production plants to meet the international requirements and the national management system to meet WHO’s standard. This is a big progress of Vietnam to contribute to bring made-in-Vietnam vaccine to the world.
The certification is the culmination of over a decade of intensive effort by the National Regulatory Authority (NRA) office to implement a roadmap — developed by national experts, with continuous advice from WHO — to strengthen capacity for regulation of vaccines.
In April of this year, a team of independent experts evaluated the Vietnam NRA for vaccines, and found that it has met all of the WHO criteria for functioning at international standards of excellence.
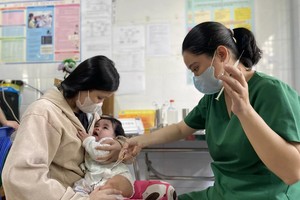
For the people of Viet Nam it means that vaccines used by Vietnam’s national immunization program are now certified safe and effective. All vaccines under the National Expanded Immunization Program are free of charge, without shortage of supply, available at commune health centers across the country.